Technical innovation in Adeno Associated Virus (AAV) vectors has exploded since the first FDA approved AAV Gene Therapy, only 6 years ago. From agriculture to electronics, you can count on technical innovation and economies of scale to drive down production costs and make products more accessible to the general public. Lower costs leading to wider patient access is the future for Gene Therapies that many are hoping for. Let’s take a look at how today’s industry benchmarks are tearing down the cost-of-goods barrier holding back AAV Gene Therapies.
The cost of producing any medicinal product is highly protected by the manufacturer and cannot be publicly known. Trying to estimate the cost of any specific AAV product is also nearly impossible as everything from production yields, capital equipment, negotiated supplier prices, and staff wages are all confidential. However with a handful of industry metrics, one can make a rough estimate for what it could cost to manufacture an AAV gene therapy. The simple calculation is as follows:

The following Table 1 lists some published references for 3 of these metrics. It’s important to keep in mind that this is a hypothetical thought exercise; this is not saying that any specific AAV production process can be optimized for every metric.
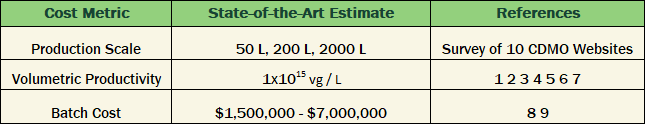
Table 1. Published industry benchmarks for key manufacturing cost metrics
Estimating the industry’s progress on lowering the manufacturing cost to treat a patient has to also consider the variable dose being administered. The doses of AAV gene therapies can be vary significantly by disease area and route of administration as published by Burdett, 2023 [10]. From Burdett, Table 3, we can look at 3 different representative doses in disease areas with commercially approved AAV gene therapies.
As shown in Figure 1 below, as the Volumetric Productivity (Titer + Yield) increases over the published range, manufacturing costs can be dramatically reduced. While the cost for manufacturing a high dose of AAV for a musculoskeletal indication is still unavoidably high ($35k-per-patient), the costs for manufacturing the lower doses used in other indications could be well under $1000-per-patient.
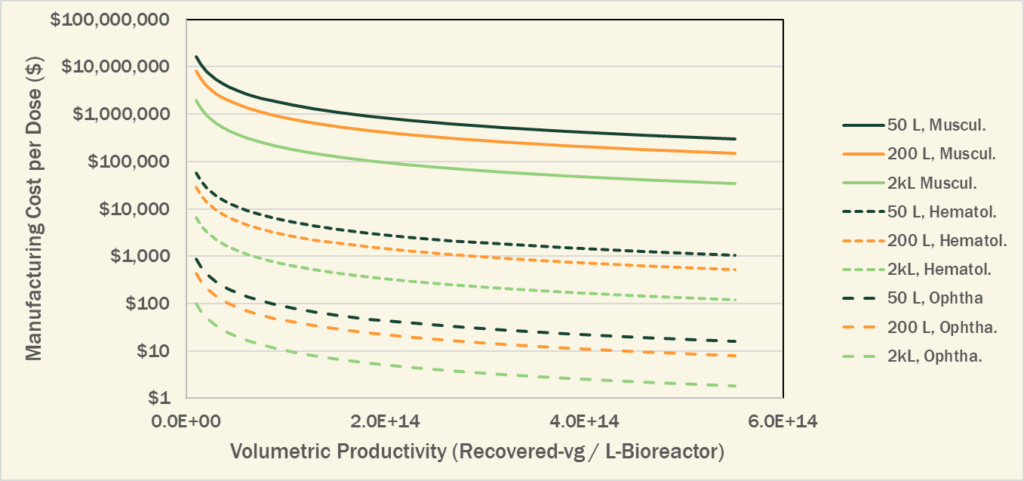
Figure 1. Reduction in manufacturing cost-per-dose through increased manufacturing productivity
A statistical summary of the average AAV dose-per-patient based on disease area or route of administration is tabulated in Burdett 2023 [10]. For the listed clinical programs, estimating the potential future commercial manufacturing costs based on today’s industry best benchmarks is shown in Tables 2 and 3 below. These cost estimates assume the following metrics:
The calculated estimates for cost-per-dose, shown in Tables 2 and 3, would suggest that manufacturing costs should become less of a problem in the near future. The cost to manufacture an average dose of AAV used in prior clinical trials translate to a fraction of the potential value of an effective gene therapy. This is a good indication for the financial viability of lower cost AAV based gene therapies across many disease areas.
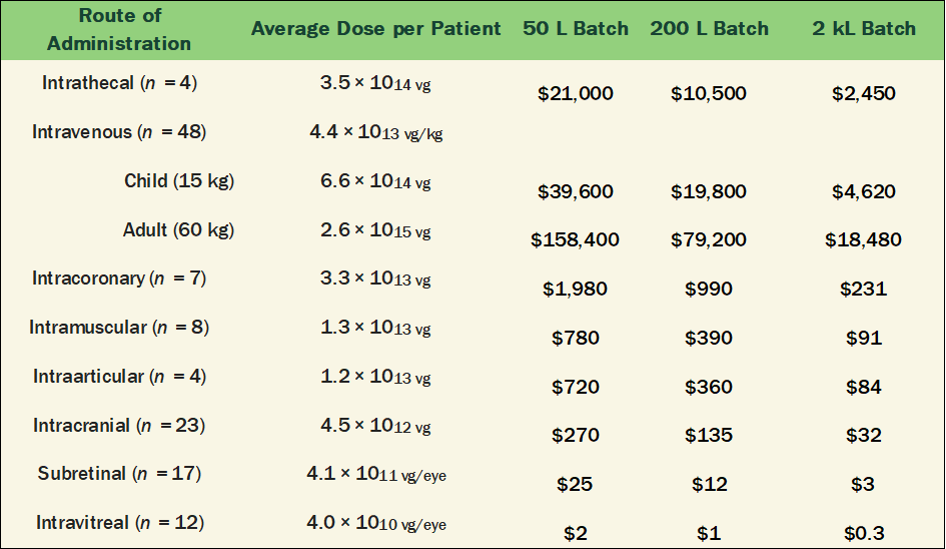
Table 2. Manufacturing Cost-per-Dose listed in Table 4 of Burdett 2023 [10] by Production Scale
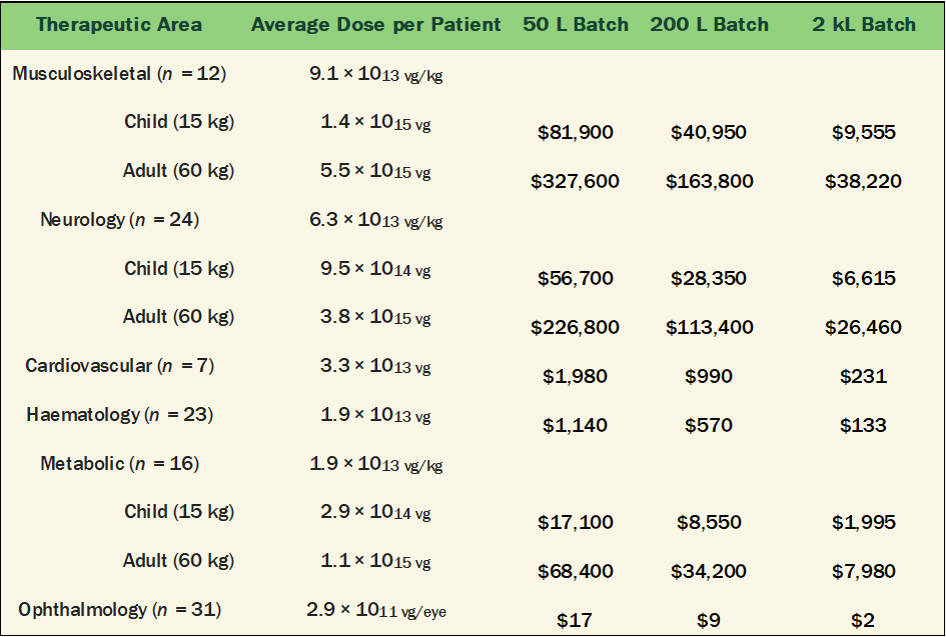
Table 3. Manufacturing Cost-per-Dose listed in Table 3 of Burdett 2023 [10] by Production Scale
The overall results are quite encouraging and demonstrate the power of technical innovation to break the tightly held belief that manufacturing AAV Gene Therapies is too expensive. All doses could be manufactured below $10k-per-patient, aside from the highest dose intravenous administration for adults with Musculoskeletal or Neurological disorders.
One very important point to also keep in mind is that manufacturing cost is only part of the total cost of administration. Many gene therapies require risky and invasive administration procedures, such as the intracranial, subretinal, and intravitreal injections. The procedural costs of administering these gene therapies, and likely the others as well, contribute to a much higher patient cost than is reflected by manufacturing the product alone.
Productivity driven cost reductions come with one big downside that has unfortunate implications for many applications of AAV gene therapies. Because batch costs remain relatively constant, or even increase over time, the cost savings is only from manufacturing more patient doses-per-equivalent-batch-cost. These cost benefits fall apart quickly if a manufacturer cannot administer or sell all the doses that have been manufactured. In both the case of running clinical trials and of ultra-rare diseases, patient numbers may be very limited. Regardless of the number of AAV doses manufactured in a single batch, in both cases the batch cost can only be burdened across the number of patients who actually receive a dose of the AAV therapy.
To look more closely at the connection between patient number and cost-per-dose, we can use a summary of AAV clinical trials published in Dobrowsky 2021 [11]. This publication lists nearly 200 AAV clinical trials, including the targeted dose and number of enrolled patients. Using the same industry manufacturing metrics, one can estimate how much manufacturing cost must be burdened onto each patient in cases where more AAV is produced than is required.
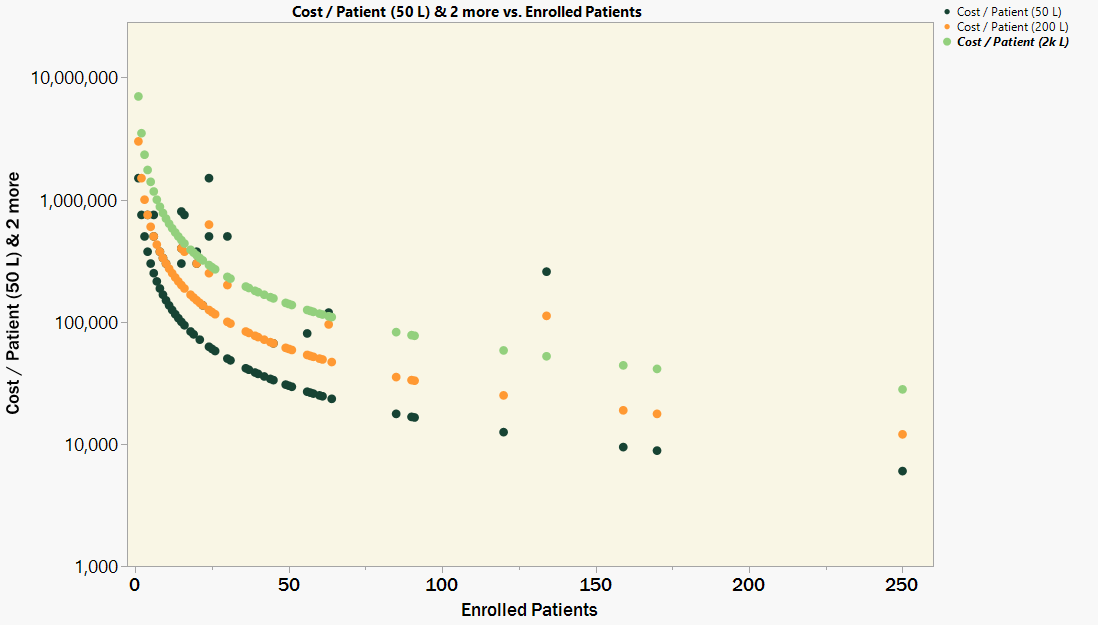
Figure 2. Manufacturing cost-per-patient when producing for very low patient numbers
In Figure 2, there is a tight correlation between the number of enrolled patients and the estimated manufacturing cost-per-patient. Aside from a few high dose trials, the dose is almost irrelevant. In cases where there are fewer than 100 patients, the manufacturing costs-per-patient increase dramatically. The burdened manufacturing cost to all patients in these scenarios, regardless of dose or disease area, is back to well above $10k.
One insight that can be drawn from this graph is that the 50 L production scale is almost universally more cost effective than the larger 200 L and 2 kL production scales. Even though the cost-per-vg is less at the larger volumes, there is no net benefit when sufficient supply can be made at a smaller and less expensive volume.
The potential for technical innovations to lower the cost barriers is just as relevant to gene therapies as every other consumer product. For high product demand prevalent diseases and large dose administrations, these productivity improvements should translate to more sustainable economic projections at a lower price to patients and payers. For indications requiring a lower dose, the lower cost-per-patient might even drive wider patient access, even into low- and middle-income countries. Across all areas and production scales, more cost effective processes will also contribute to lower upfront research and development costs too.
These manufacturing cost projections also shed light on the challenges to control costs when working in the area of rare disease. There are diminishing cost benefits with increasing total volumetric productivity in the context of low patient numbers. Alternative strategies for reducing the overall batch cost, by reducing capital overhead, labor, and materials/consumables cost, may become more effective.
Innovations enabling smaller batches that require less capital and labor overhead are the way to go. Antithetical to decentralized business models, centralized manufacturing where many smaller batches can produced in parallel could be more capital efficient but only if equipment and process standards are adhered to. However maintaining the consistent flow of low demand products on a standard platform could be achieved in a collaborative Decentralized Science ecosystem.
